Tonix Pharmaceuticals Holding Corp. - Common Stock (TNXP)
14.43
-0.41 (-2.76%)
NASDAQ · Last Trade: Feb 26th, 7:05 PM EST
Detailed Quote
| Previous Close | 14.84 |
|---|---|
| Open | 14.81 |
| Bid | 14.40 |
| Ask | 14.90 |
| Day's Range | 14.25 - 15.03 |
| 52 Week Range | 6.760 - 69.97 |
| Volume | 450,926 |
| Market Cap | 169.94M |
| PE Ratio (TTM) | 0.0517 |
| EPS (TTM) | 279.3 |
| Dividend & Yield | N/A (N/A) |
| 1 Month Average Volume | 345,097 |
Chart
About Tonix Pharmaceuticals Holding Corp. - Common Stock (TNXP)
Tonix Pharmaceuticals Holdings is a clinical-stage biopharmaceutical company focused on developing innovative therapies for central nervous system disorders and other serious medical conditions. The company is dedicated to advancing its pipeline of product candidates, which include treatments for conditions such as migraines, post-traumatic stress disorder, and various infectious diseases. By leveraging its expertise in drug development and utilizing strategic collaborations, Tonix aims to bring novel treatment options to market that address unmet medical needs and improve patient outcomes. Read More
News & Press Releases
BioMedNewsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) to Participate in March Investor Conferences
Tonix Pharmaceuticals (NASDAQ: TNXP) announced that its management team will participate in two upcoming investor conferences in March 2026. Seth Lederman, M.D., president and chief executive officer, will deliver a company presentation at the TD Cowen 46th Annual Healthcare Conference on Wednesday, March 4, 2026, from 11:10 a.m. to 11:40 a.m. ET at the Boston Marriott Copley Place in Boston. In addition, management will participate in one-on-one meetings at the Barclays 28th Annual Healthcare Conference, scheduled for March 10-12, 2026, at the Loews Miami South Beach Hotel in Miami Beach.
Via Investor Brand Network · February 25, 2026
TinyGemsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) Presents Positive Phase 3 Data for TONMYA(TM) in Fibromyalgia at Non-Opioid Pain Summit
Tonix Pharmaceuticals (NASDAQ: TNXP) announced the presentation of clinical data for TONMYA(TM), previously investigated as TNX-102 SL, at the 2026 Non-Opioid Pain Therapeutics Summit held Jan. 29, 2026, in Boston. The data were drawn from RESILIENT, a 14-week Phase 3 randomized, double-blind, placebo-controlled trial involving 456 patients with fibromyalgia, in which bedtime sublingual administration of TONMYA demonstrated a statistically significant reduction in weekly average pain scores at Week 14 versus placebo (p<0.0001), along with significant improvements in sleep disturbance, fatigue, and functional outcomes. Tonix reported that TONMYA was well tolerated, with low discontinuation rates and primarily mild, self-limited adverse events, supporting its potential as a differentiated, centrally acting non-opioid treatment designed to address both pain and non-restorative sleep in fibromyalgia patients.
Via Investor Brand Network · January 30, 2026
BioMedNewsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) to Present at January Investor Conferences
Tonix Pharmaceuticals (NASDAQ: TNXP) announced that its management team will present and host investor meetings at multiple January investor conferences, including the Sachs Associates 9th Annual Neuroscience Innovation Forum and Biotech Showcase 2026, both held in San Francisco. President and Chief Executive Officer Seth Lederman, M.D., is scheduled to deliver a company presentation and participate in a panel discussion focused on neuropsychiatric drug development at the Sachs Associates forum, and to present at Biotech Showcase 2026, with a replay of the presentation expected to be made available on the company’s website following the event.
Via Investor Brand Network · January 6, 2026
InvestorNewsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) to Present at January Investor Conferences
Tonix Pharmaceuticals (NASDAQ: TNXP) announced that its management team will present and host investor meetings at multiple January investor conferences, including the Sachs Associates 9th Annual Neuroscience Innovation Forum and Biotech Showcase 2026, both held in San Francisco. President and Chief Executive Officer Seth Lederman, M.D., is scheduled to deliver a company presentation and participate in a panel discussion focused on neuropsychiatric drug development at the Sachs Associates forum, and to present at Biotech Showcase 2026, with a replay of the presentation expected to be made available on the company’s website following the event.
Via Investor Brand Network · January 6, 2026
This Pharma Stock Shot Up 4% Pre-Market After FDA Approval For Drug, Retail’s Super Bullish But Believes ‘This Is A Marathon, Not A Sprint’stocktwits.com
Via Stocktwits · August 18, 2025

The company also announced the pricing of a $20 million registered direct offering.
Via Stocktwits · December 29, 2025
InvestorNewsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) Provides Update on TNX-4800 Lyme Disease Prophylaxis Program
Tonix Pharmaceuticals (NASDAQ: TNXP) provided program updates on TNX-4800, a long-acting human monoclonal antibody designed for seasonal prophylaxis against Lyme disease, which targets the outer surface protein A of Borrelia burgdorferi, the bacterium that causes Lyme disease. The company said TNX-4800 is being developed as a once-yearly subcutaneous administration intended to provide protection throughout the U.S. tick season and noted there are currently no FDA-approved vaccines or prophylactics for Lyme disease. Tonix plans to meet with the FDA in 2026 to discuss Phase 2/3 development options, including the potential use of a controlled human infection model, and expects to have GMP-manufactured investigational product available for testing in early 2027.
Via Investor Brand Network · December 29, 2025
TinyGemsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) Announces $20 Million Registered Direct Offering with Point72
Tonix Pharmaceuticals (NASDAQ: TNXP) announced it has entered into a securities purchase agreement with Point72 for a registered direct offering of 615,025 shares of common stock at $16.26 per share, or pre-funded warrants to purchase up to an aggregate of 615,025 shares at a purchase price of $16.259 per warrant, for gross proceeds of approximately $20.0 million before fees and expenses. The offering is expected to close on or about Dec. 30, 2025, subject to customary closing conditions, with net proceeds intended to fund commercialization of marketed products, development of the company’s product pipeline, and general working capital and corporate purposes. TD Cowen is acting as sole placement agent, with A.G.P./Alliance Global Partners serving as financial advisor.
Via Investor Brand Network · December 29, 2025
InvestorNewsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) Appoints Irina Ishak as General Counsel
Tonix Pharmaceuticals (NASDAQ: TNXP), a fully integrated commercial-stage biotechnology company, appointed Irina Ishak as General Counsel effective Dec. 8, 2025. Ishak, a longtime advisor to Tonix, will lead the Company’s legal, corporate governance and compliance functions. She joins from Lowenstein Sandler LLP, where she served as Senior Counsel and advised Tonix on financings, licensing, strategic transactions, commercial agreements and governance matters. Ishak previously held senior legal roles at Savient Pharmaceuticals, contributing to the development and launch of KRYSTEXXA (R). CEO Seth Lederman said her corporate and transactional experience will support Tonix as it commercializes products, advances its pipeline and executes long-term strategy. Ishak said she is honored to join Tonix during a pivotal period following the launch of the first FDA-approved fibromyalgia therapy in more than 15 years.
Via Investor Brand Network · December 9, 2025

AUSTIN, Texas, Dec. 04, 2025 (GLOBE NEWSWIRE) -- BioMedWire Editorial Coverage: Chronic illnesses and rare disorders among older Americans represent a rapidly intensifying challenge for the U.S. healthcare system, with the National Institutes of Health estimating that more than 30 million people nationwide are living with a rare disease. Most of these conditions have no FDA-approved therapies, leaving older adults particularly at risk as age-related changes can mask or delay accurate diagnosis. This growing strain on care has heightened the need for novel treatments that address significant unmet medical needs. Soligenix Inc. (NASDAQ: SNGX) (Profile), a late-stage biopharmaceutical developer, is advancing several rare-disease therapies, including HyBryte[TM] (synthetic hypericin) for cutaneous T-cell lymphoma (CTCL), where it is currently running the final confirmatory trial necessary before seeking global marketing authorization. As the Trump administration pursues new health-policy measures focused on chronic and rare diseases, Soligenix’s programs are positioned at a pivotal crossroads of scientific innovation and national healthcare goals. The company is working alongside several prominent leaders in the pharmaceutical and life sciences sectors, including AMGEN Inc. (NASDAQ: AMGN), Amicus Therapeutics Inc. (NASDAQ: FOLD), Citius Oncology Inc. (NASDAQ: CTOR) and Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP).
By BioMedWire · Via GlobeNewswire · December 4, 2025

Retail traders pointed to the expanded buyback authorization as a sign of management confidence, framing it as a potential catalyst for renewed valuation upside.
Via Stocktwits · November 18, 2025

Tonix Pharmaceuticals on Monday announced the launch of its new drug to treat Fibromyalgia.
Via Stocktwits · November 17, 2025

Tonix Pharmaceuticals shares are rising Monday morning after the company announced the U.S. commercial availability of its new drug, TONMYA.
Via Benzinga · November 17, 2025

Tonix Pharmaceuticals stock surged 77% after Q3 earnings beat estimates and confirming the November launch of its new fibromyalgia drug, Tonmya.
Via Chartmill · November 10, 2025
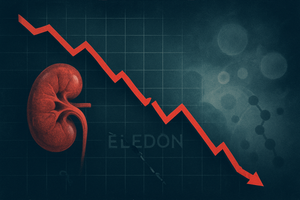
San Diego, CA – November 10, 2025 – Eledon Pharmaceuticals (NASDAQ: ELDN) witnessed a dramatic collapse in its stock price, shedding nearly 50% of its value following the announcement of mixed results from its pivotal Phase 2 BESTOW clinical trial for tegoprubart. The drug, aimed at preventing organ rejection in kidney transplant
Via MarketMinute · November 10, 2025
InvestorNewsBreaks – Tonix Pharmaceuticals Holding Corp. (NASDAQ: TNXP) Advances TNX-2900 for Prader-Willi Syndrome Into Phase 2 Trial
Tonix Pharmaceuticals (NASDAQ: TNXP) announced that the FDA has cleared its Investigational New Drug application to begin a Phase 2 trial of TNX-2900, a proprietary magnesium-potentiated intranasal oxytocin formulation for Prader-Willi syndrome (PWS), a rare genetic disorder and leading cause of life-threatening childhood obesity; the program has received Orphan Drug and Rare Pediatric Disease designations, making Tonix eligible for a transferable Priority Review Voucher upon approval.
Via Investor Brand Network · September 30, 2025

Via Benzinga · August 19, 2025

Intrigued by the market activity one hour before the close of the markets on Monday? Uncover the key winners and losers of today's session in our insightful analysis.
Via Chartmill · August 18, 2025

Via Benzinga · August 18, 2025

Via Benzinga · August 18, 2025

Curious to know what's happening on the US markets in the middle of the day on Monday? Join us as we explore the top gainers and losers in today's session.
Via Chartmill · August 18, 2025

Let's have a look at the gap up and gap down stocks in today's session.
Via Chartmill · August 18, 2025

FDA approves Tonmya, a non-opioid fibromyalgia therapy from Tonix, with strong trial data and U.S. launch expected in late 2025.
Via Benzinga · August 18, 2025
Via Benzinga · August 18, 2025

Via Benzinga · August 15, 2025
